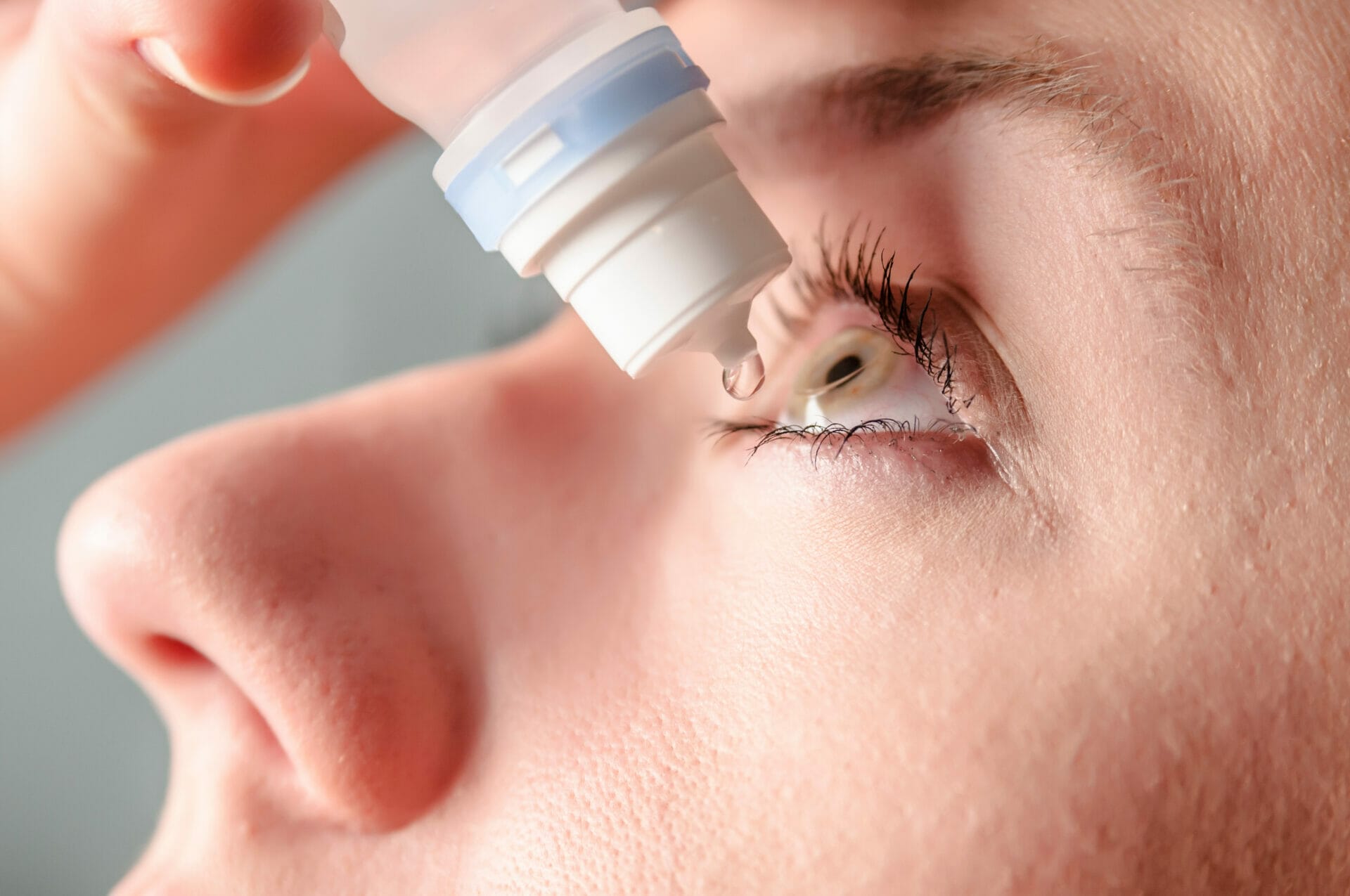
We are currently litigating against the manufacturers of EzriCare and Delsam Pharma’s Artificial Tears in the US. This week, Bloomberg published a feature on how the eyedrops, made in an Indian factory and tainted with an antibiotic-resistant superbug (pseudomonas aeruginosa), slipped past the FDA.
The contamination is believed to have originated in the manufacturing plant in India as a result of deficiencies in the process control standards. After discovering the issue, the Centers for Disease Control and Prevention (CDC) recommended that the eyedrops be recalled and removed from the market.
The ‘unusually virulent strain’ of pseudomonas aeruginosa was found in people’s lungs and in their blood. It appeared in the US in May 2022, starting in Los Angeles before spreading through Connecticut and Utah.
It took the CDC eight months to ‘determine the culprit’, which was found to be EzriCare and Desalm Pharma’s artificial tears.
Bloomberg Report on EzriCare Artificial Tears
Bloomberg reported: “The two affected brands were widely available for less than half the price of better-known ones. They weren’t counterfeit. They weren’t imported illegally. They were made in India and sold by two US distributors in boxes stamped with the drug inventory numbers that the Food and Drug Administration issued.
“The eyedrops from EzriCare LLC and Delsam Pharma LLC were recalled after the pseudomonas contributed to four deaths, 18 cases of vision loss and scores of infections.
“The particulars are horrific. In four people, the bacteria spread so quickly that doctors had to remove their eyeballs to stop it. Others had their corneas turn into cloudy abscesses of scar tissue. Some endured migraines, discharges from their eyes and light sensitivity that kept them in the dark for days and even months.”
According to Bloomberg, sufficient tests ensuring the safety of products were not completed, and it was the ‘gaping hole in the FDS’s supervision of over-the-counter medicines’ that made the outbreak possible.
Instead of taking responsibility for the aftereffects of the faulty products, the companies involved are now passing the blame.
Comment from Pogust Goodhead
Mike Daly, Partner at Pogust Goodhead, said: “It’s truly worrying that it appears the FDA has very little idea about what’s coming into the country, and there were no tests or inspections to ensure that the products and manufacturing facilities were safe for American citizens.
“Even more concerning is that these contaminated products can be quickly, cheaply, and widely spread across our country by giant retailers like Amazon and Walmart, who also appear to have failed to employ safeguards that may have stopped the spread of the infectious products. We suspect the trauma, fear, and pain caused by these contaminated eye drops will endure for some time.”
Pogust Goodhead is taking Global Pharma, EzriCare, and Amazon to court in New Jersey. We will also be filing on behalf of numerous other individuals from around the US who suffered horrific vision loss and eye damage.
As a global law firm, we are committed to litigating these cases in the US and have begun pursuing potential new claims for individuals in similar circumstances all across the world, as similar catastrophes caused by manufacturing facilities in India have also wreaked havoc on children and communities in Indonesia, Gambia, and elsewhere.
You can read the full Bloomberg article here. If you or someone you know has been affected by this case, please register your interest in our potential lawsuit and a member of our legal team will assess your claim.