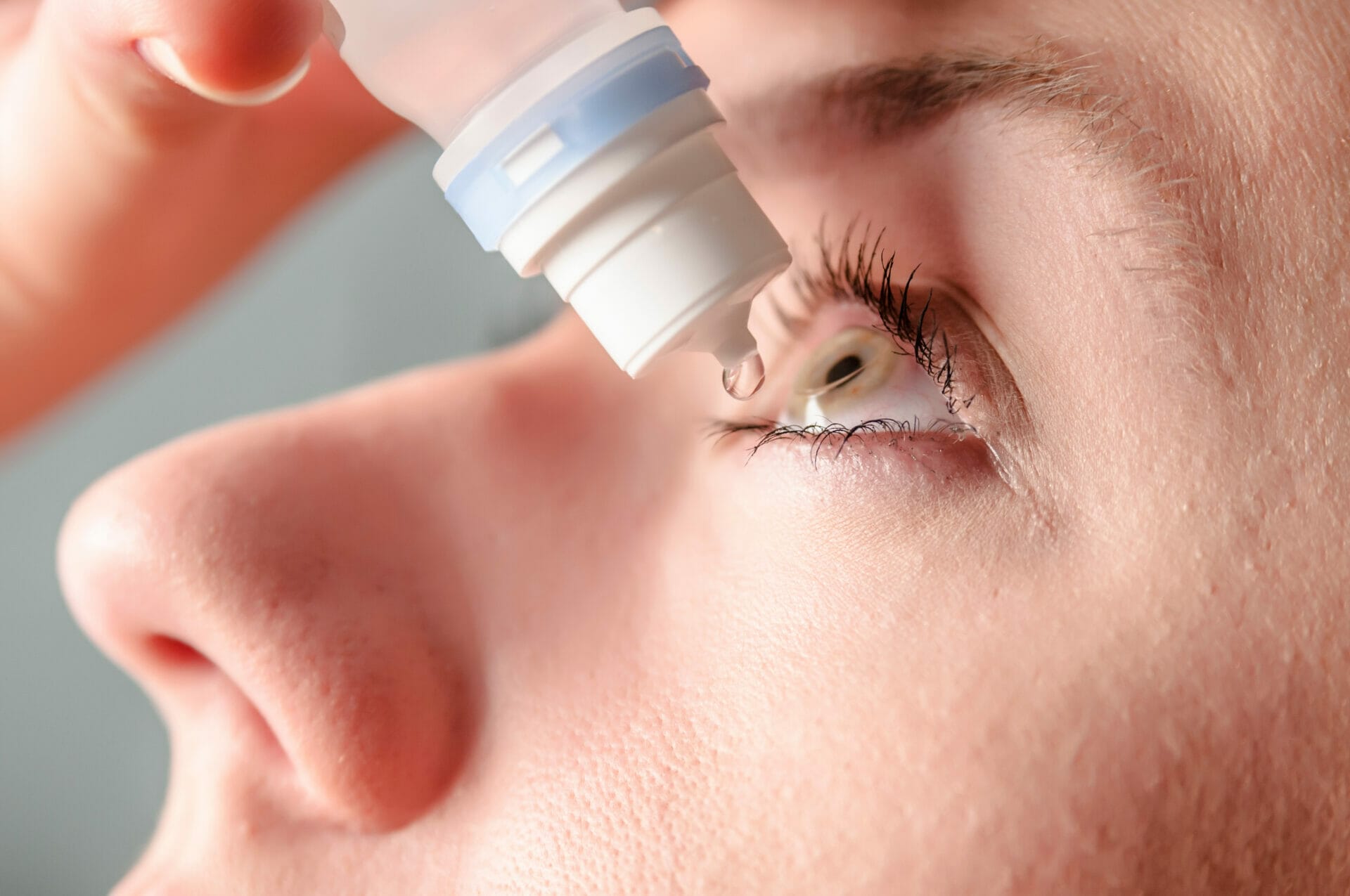
Pogust Goodhead is currently investigating serious side effects from eye drops after federal health officials in America warned against 26 over-the-counter eye care products that could cause infections and blindness.
On October 30, the Food and Drug Administration (FDA) advised consumers to avoid a list of products sold at major retailers including CVS, Rite Aid, Target, and Walmart, due to potential contamination risks that could result in eye infections and, in severe cases, partial vision loss or blindness.
Eyedrops must be sterile because applying them to the eyes bypasses the body’s natural defense mechanisms, however, FDA investigators uncovered “ unsanitary conditions” in a manufacturing plant responsible for producing these products. Bacterial tests conducted in critical drug production areas signaled a potential health risk.
The FDA is urging healthcare professionals and patients to report any cases of infection that may be related to these products.
Major retailers are removing the affected products from their shelves and online platforms, but some products may still be available in stores under the brand names of a Leader, Rugby, and Velocity.
Pogust Goodhead eye drops lawsuit
The warning follows other recent issues in the Ophthalmic drug product market.
In January, the CDC and FDA warned against the use of EzriCare Artificial Tears which were linked to drug-resistant bacteria, causing numerous fatalities and vision loss.
The contamination was believed to have originated in the manufacturing plant, where deficiencies in the process control standards can result in contamination of the product with microorganisms.
In 2023, Pogust Goodhead filed a lawsuit against Ezricare to help those who have been affected by the product seek compensation.
Keeping consumers safe
By 2024, 123 million Americans are expected to use eyedrops according to Statista, making it crucial for consumers to stay informed and prioritize product safety.
If you have purchased or used any of these products, it is strongly recommended you stop using them immediately and get in touch to discuss your legal rights.
Our Mass Torts department has extensive experience representing people across the world who were harmed by unsafe products, pharmaceuticals, and medical devices. Our team is committed to safeguarding your rights and interests.
If you or someone you know has purchased or been injured by an eye care product listed below, you can get in touch with our team by emailing intake@pogustgoodhead.com or calling (610) 941-4204 for a free, confidential conversation.
Eye drop product recall details
Eye Drop Retailers and Product Information
| Retailer/ Label | Product | Product Information |
| CVS Health | Lubricant Eye Drops 15 ml (single pack) | Carboxymethylcellulose Sodium Eye Drops 0.5% w/v |
| Lubricant Eye Drops 15 ml (twin pack) | Carboxymethylcellulose Sodium Eye Drops 0.5% w/v | |
| Lubricant Gel Drops 15 ml (single pack) | Carboxymethylcellulose Sodium Eye Drops 1% w/v | |
| Lubricant Gel Drops 15 ml (twin pack) | Carboxymethylcellulose Sodium Eye Drops 1% w/v | |
| Multi-Action Relief Drops 15 ml | Polyvinyl Alcohol 0.5% w/v & Povidone 0.6% w/v & Tetrahydrozoline Hydrochloride 0.05% Eye Drops | |
| Lubricating Gel drops 10 ml | Polyethylene Glycol 400 0.4% & Propylene Glycol 0.3% Eye Drops | |
| Lubricant Eye Drops 10 ml (single pack) | Propylene Glycol Eye Drops 0.6% w/v | |
| Lubricant Eye Drops 10 ml (twin pack) | Propylene Glycol Eye Drops 0.6% w/v | |
| Mild Moderate Lubricating Eye Drops 15 ml (single pack) | Polyethylene Glycol 400 Eye Drop ‘0.25% w/v | |
| Rugby (Cardinal Health) | Lubricating Tears Eye Drops 15 ml | Hypromellose 2910-0.3% w/v & Dextran 70- 0.1% Eye Drops |
| Polyvinyl Alcohol 1.4% Lubricating Eye Drops 15 ml | Polyvinyl Alcohol Eye Drops 1.4% w/v | |
| Leader (Cardinal Health) | Dry Eye Relief 10 ml | Polyethylene Glycol 400 0.4% & Propylene Glycol 0.3% Eye Drops |
| Lubricant Eye Drops 15 ml (single pack) | Carboxymethylcellulose Sodium Eye Drops 0.5% w/v | |
| Lubricant Eye Drops 15 ml (twin pack) | Carboxymethylcellulose Sodium Eye Drops 0.5% w/v | |
| Dry Eye Relief 15 ml | Carboxymethylcellulose Sodium Eye Drops 1% w/v | |
| Eye Irritation Relief 15 ml | Polyvinyl Alcohol 0.5% w/v & Povidone 0.6% w/v & Tetrahydrozoline Hydrochloride 0.05% Eye Drops | |
| Rite Aid | Lubricant Eye Drops 15 ml (twin pack) | Carboxymethylcellulose Sodium Eye Drops 0.5% w/v |
| Lubricant Eye Drops 10 ml (twin pack) | Propylene Glycol Eye Drops 0.6% w/v | |
| Gentle Lubricant Gel Eye Drops 15 ml | Hypromellose 0.3%, Glycerin 0.2%, Dextran 70 0.1% Eye Drops | |
| Lubricant Gel Drops 15 ml | Carboxymethylcellulose Sodium Eye Drops 1% w/v | |
| Lubricating Gel Drops 10 ml | Polyethylene Glycol 400 0.4% & Propylene Glycol 0.3% Eye Drops | |
| Multi-Action Relief Drops 15 ml | Polyvinyl Alcohol 0.5% w/v & Povidone 0.6% w/v & Tetrahydrozoline Hydrochloride 0.05% Eye Drops | |
| Target | Up&Up Dry Eye Relief Lubricant Eye Drops 30 ml | Polyethylene Glycol 400 0.4% & Propylene Glycol 0.3% Eye Drops |
| Up&Up Extreme Relief Dry Eye 15 ml (single pack) | Polyethylene Glycol 400 0.4% & Propylene Glycol 0.3% Eye Drops | |
| Up&Up Extreme Relief Dry Eye 30 ml (twin pack) | Carboxymethylcellulose Sodium Eye Drops 0.5% w/v | |
| Velocity Pharma LLC | Lubricant Eye Drop 10 ml (triple pack) | Propylene Glycol Eye Drops 0.6% w/v |
| Walmart | Equate Hydration PF Lubricant Eye Drop 10 ml | Polyethylene Glycol 400 0.4% & Propylene Glycol 0.3% Eye Drops |