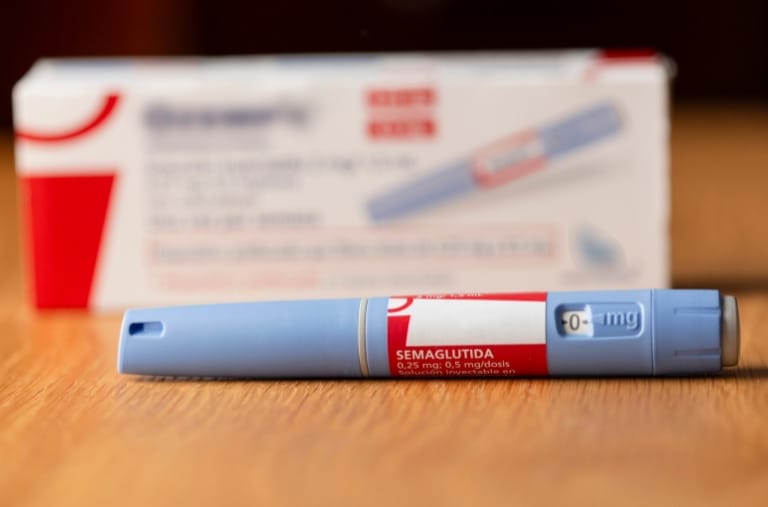
Pogust Goodhead is currently investigating serious side effects that patients have experienced due to the type 2 diabetes and weight loss drugs Ozempic and Wegovy.
In recent years, Wegovy and Ozempic, drugs initially approved to manage type 2 diabetes and promote weight loss, have gained immense popularity. But beneath their seemingly miraculous results lies a series of serious side effects which have led to legal actions against its manufacturers.
Wegovy and Ozempic have been touted as a breakthrough in diabetes management and quickly become household names.
Ozempic’s approval in 2017 marked a turning point in diabetes treatment, however, its popularity led to off-label use, especially for weight loss purposes.
Aggressive marketing strategies, the cosign from countless celebrities and substantial financial investments in advertising and promotions, contributed to its widespread adoption, making it one of the most prescribed drugs in the world.
Ozempic controversy
In August 2023, a significant legal development unfolded in the U.S. District Court for the District of Louisiana. A complaint was filed against Novo Nordisk, the Danish manufacturer of Ozempic, and Eli Lilly, the creator of Mounjaro, another drug that works as a GLP-1 receptor agonist.
The complaint alleged that the drugs increase the risk of gastroparesis and gastroenteritis, leading to severe consequences such as abdominal pain, vomiting undigested food, acid reflux, and more.
Despite various case studies and clinical reports warning about these risks, the manufacturers had not updated their label warnings, leaving patients unaware of the potential dangers.
Ozempic and Wegovy side effects
Ozempic and Wegovy have been linked to several severe side effects such as gastroparesis and gastroenteritis, these conditions are characterized by stomach paralysis and inflammation, causing significant discomfort and health complications. The use of these drugs has also been linked to deep vein thrombosis and aspiration, posing life-threatening risks to patients.
Legal recourse for patients who took Wegovy and Ozempic
While Wegovy and Ozempic promised hope for diabetes management and weight loss, the serious side effects associated their usage have raised significant concerns. Legal actions are underway to seek justice for those affected, emphasizing the importance of understanding the potential risks associated with pharmaceutical drugs.
Individuals who have experienced injuries or complications linked to their use of Wegovy and Ozempic are urged to seek legal advice.
Pogust Goodhead’s Mass Torts department, headquartered in Philadelphia, Pennsylvania, has extensive experience representing people across the world who were harmed by unsafe products, pharmaceuticals, and medical devices.
If you or someone you know has developed severe injuries or complications due to the use of Ozempic or Wegovy, please contact us today for a completely free and confidential conversation.
You can get in touch with our team by emailing intake@pogustgoodhead.com or calling (610) 941-4204.